

Our Science
Targeted Protein Degradation
Why TPD?
Protein degradation offers advantages to improve clinical outcomes:
- Target enzymatic and non-enzymatic roles
- Opportunity to target less conserved regions of the protein of interest vs related family members
- Target historically “undruggable” proteins
- Catalytic mechanism reduces the drug concentration required at site of action
Targeted Protein Degradation
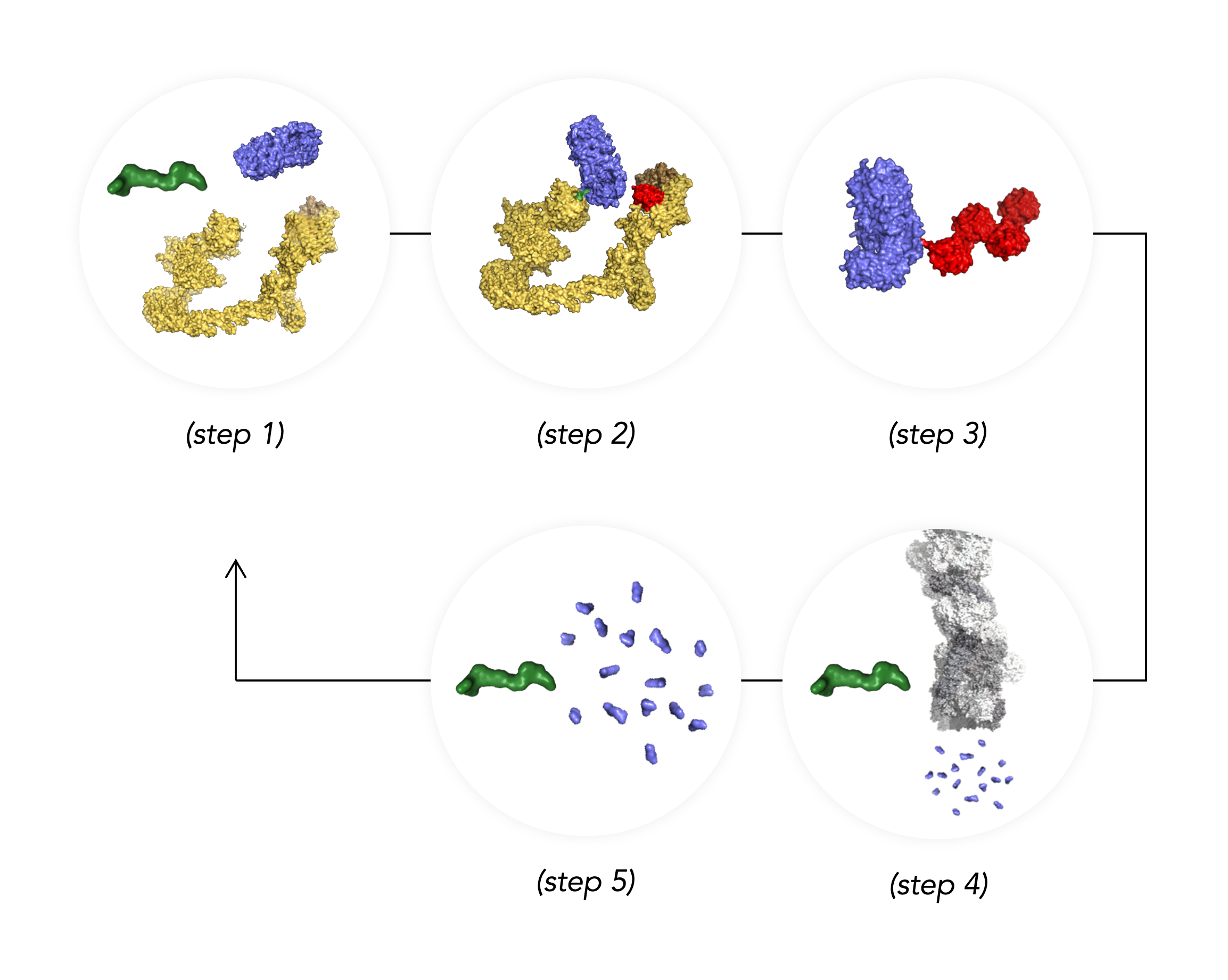
The Ubiquitin Proteasome (UPS) is responsible for the critical cellular process of protein degradation, essential for maintaining cellular homeostasis. This process can be hijacked to remove disease causing proteins.
A drug-like protein degrader (step 1) binds a target Protein of Interest (POI) and an E3 to bring them together resulting in the formation of a ternary complex (step 2). This facilitates the attachment of multiple ubiquitin tags to the POI (step 3) marking it for rapid degradation by the Proteasome (step 4). The degrader survives this process (step 5) allowing it to target additional copies of the same POI for destruction.
SK Life Science Labs utilizes this targeted approach to design degrader medicines that will treat debilitating diseases to improve the lives of patients.
MOLECULAR KEY
*Molecules in Key and Figures are not to scale

Our Pipeline
SK Life Science Labs’ pipeline and platforms are well-positioned for partnering opportunities.
| Compound | Indication |
Discovery
Preclinical
|
|---|---|---|
| p300 |
Oncology
|
|
| Heterobifunctional Target |
Oncology
|
|
| Molecular Glue Target |
Oncology
|
Partnership opportunities
| TARGET | INDICATION |
| IKZF2 (PVTX-405 DC candidate) | Solid tumors |
| ER (PVTX-321 DC candidate) | HR+ breast cancer |
| STAT3 | Immunology and Oncology |
| SMARCA2 | Solid tumors |
Therapeutic Strategy for Cancer
At SK Life Science Labs, we are dedicated to the discovery and development of degrader medicines to improve the lives of patients. SK Life Science Labs has discovered selective heterobifunctional molecules that can selectively degrade p300. We believe this approach will demonstrate significant therapeutic benefit in a number of tumor types.
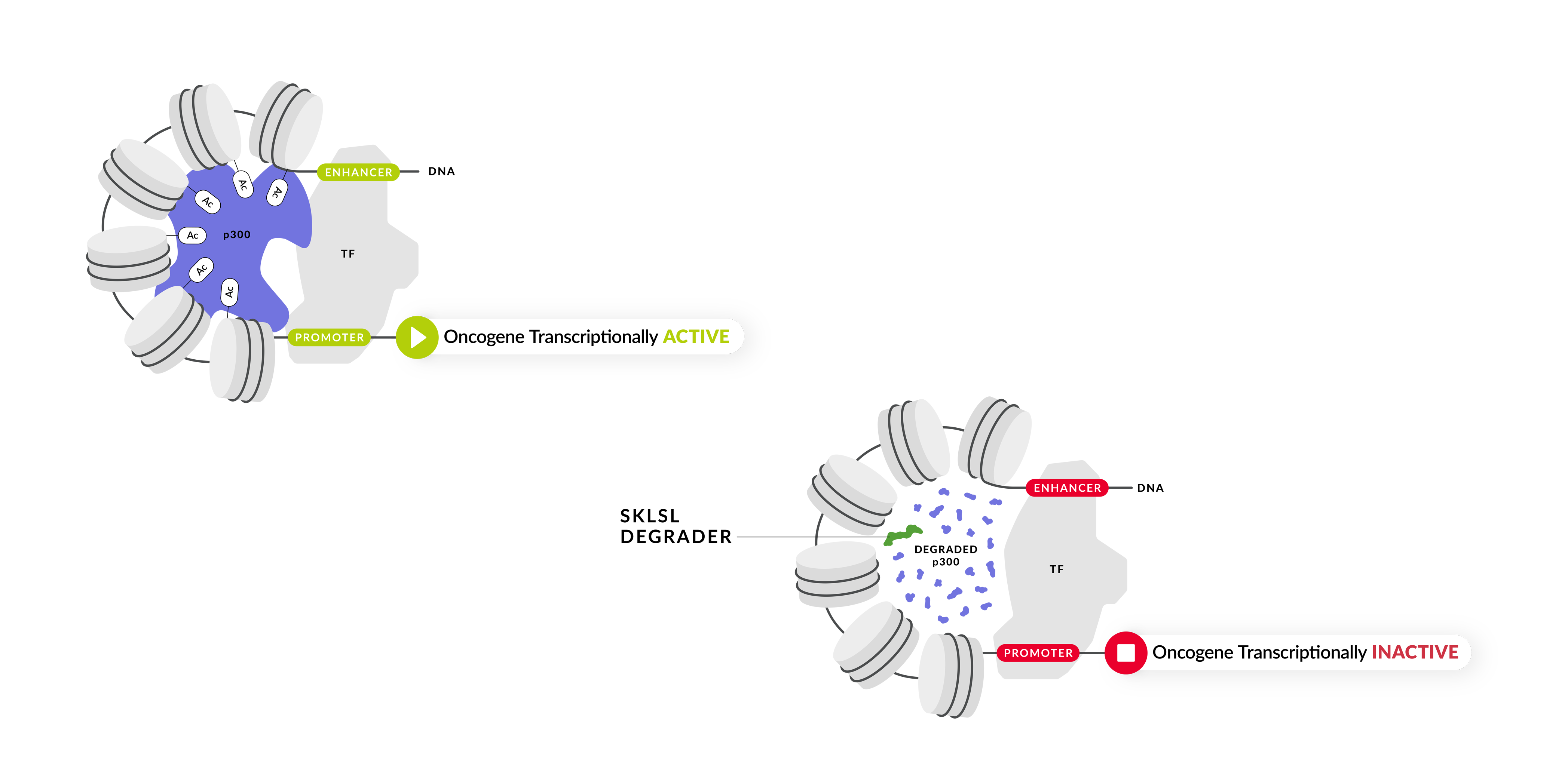
The p300 histone acetyltransferase is implicated in oncogenic processes that drive a variety of solid cancers. p300 functions as a coactivator of many transcription factors by acetylating histones and allowing for the transcription of many genes including oncogenic drivers. Selective degradation of p300 results in removal of histone acetylation marks, inactivates transcription, and thereby repression of key oncogenes known to promote tumorigenesis. p300 degradation leads to significant reduction of tumor growth in preclinical models.
MOLECULAR KEY
*Molecules in Key and Figures are not to scale

Select For More Information on P300 SELECTIVE DEGRADER PROGRAM
A first-in-class asset with tremendous therapeutic potential in oncology
- Orally bioavailable, highly selective compounds that demonstrate sub-nanomolar p300 degradation
- Degrading p300 results in repression of histone acetylation, repression of gene expression, and growth inhibition
- Significantly less toxicity than dual p300/CBP suppression in preclinical hematopoietic progenitor assays
- AR+ prostate cancer cell growth inhibited by p300 degraders
- Degradation of p300 results in a suppression of AR-mediated gene signatures
- Significant anti-tumor activity in AR+ prostate cancer mouse models is evident
- Refined biomarker predicts CBP loss-of-function (LoF) mutations across a number of tumor types
- Degradation of p300 results in selective cell growth inhibition in predicted LoF models across cancers
- In vivo growth inhibition in a CBP LoF tumor model
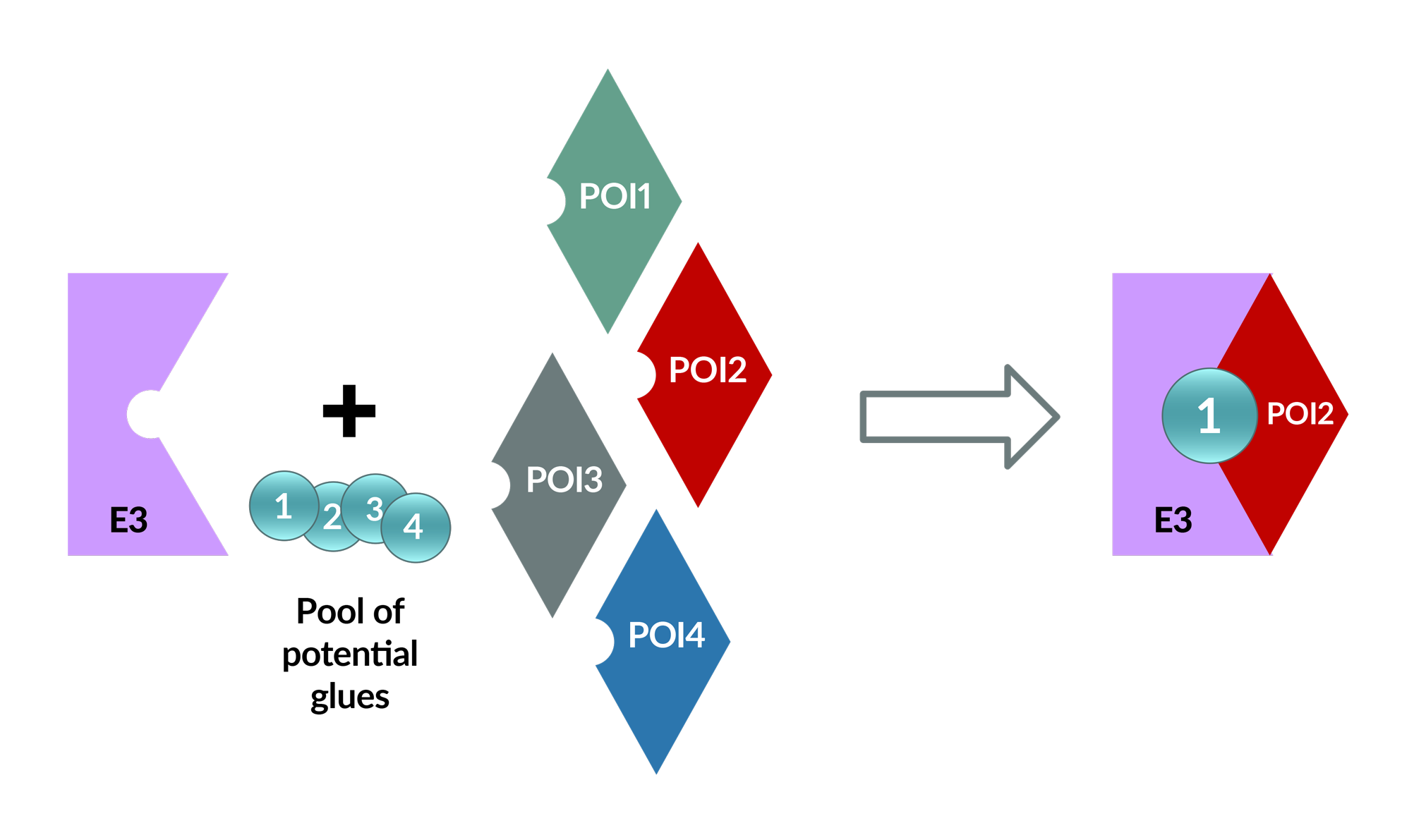
MOPED™
SK life Science Labs expands access to target biology accessible by TPD. Our MOlecular Proximity Enabled Detection (MOPED™) platform uses an exquisitely sensitive induced proximity assay to discover glues between targets and E3s. With a library of >20 E3s, glue hits for multiple targets have been discovered.